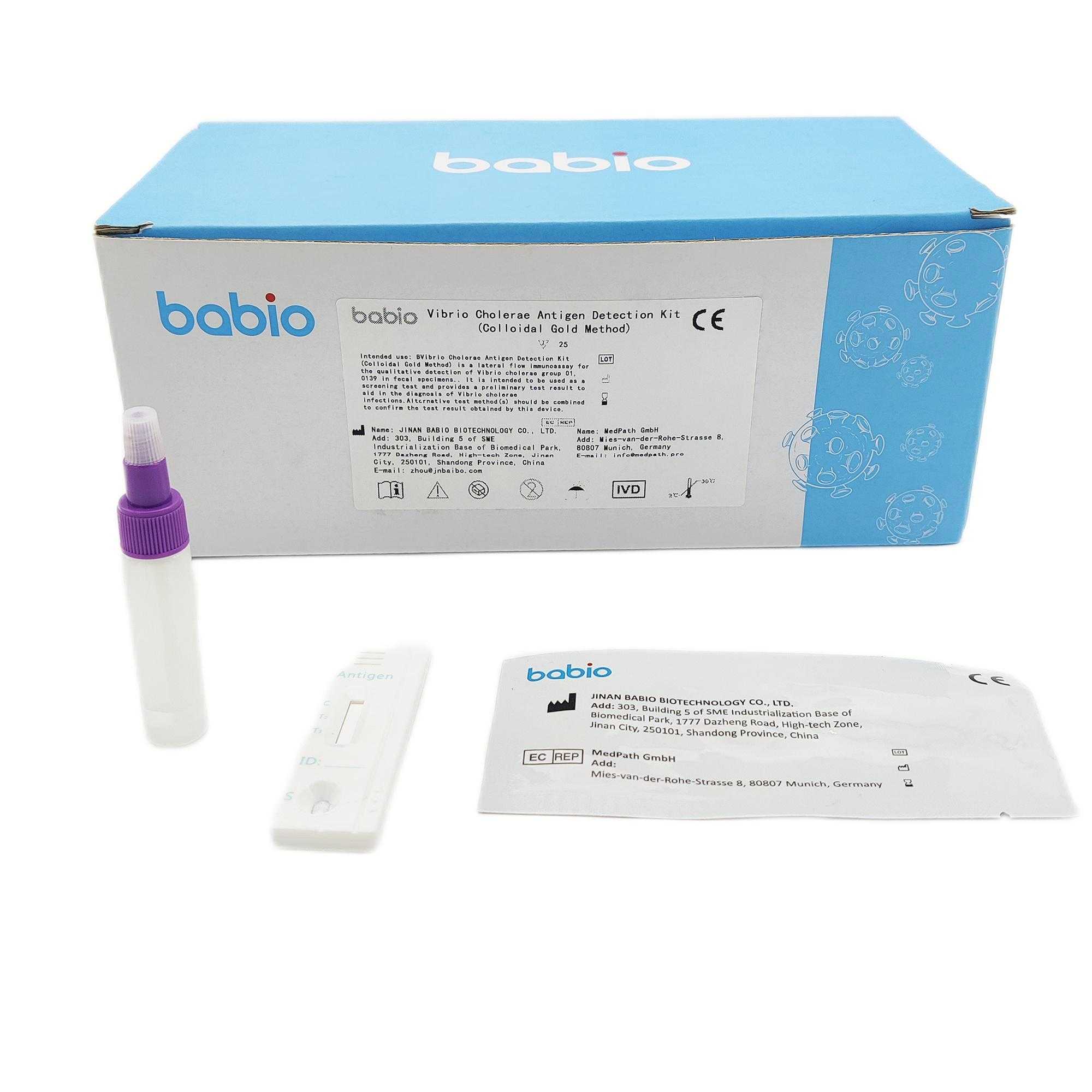
INTENDED USE
Vibrio Cholerae Antigen Detection Kit (Colloidal Gold Method) is a lateral flow immunoassay for the qualitative detection of Vibrio cholerae group 01, 0139 in fecal specimens. It is intended to be used as a screening test and provides a preliminary test result to aid in the diagnosis of Vibrio cholerae infections.
Any interpretation or use of this preliminary test result must also rely on other clinical findings as well as on the professional judgment of health care providers. Alternative test method(s) should be combined to confirm the test result obtained by this device.
Vibrio cholerae is the pathogen of human cholera, which is one of the ancient and widespread severe infectious diseases. It has caused many pandemics in the world, mainly manifested as severe vomiting, diarrhea, water loss, and high mortality. It is an international quarantinable infectious disease. Vibrio Cholerae Antigen Detection Kit (Colloidal Gold Method) can provide rapid detection of Antigens of Vibrio cholerae 01, 0139 from symptomatic patients.It can provides an instant test result in 15 minutes by minimally skilled personnel without the use of laboratory equipment.
1. Open the packaging box, take out the inner package and let it equilibrate to room temperature.
2. Remove the test card from sealed pouch and use within 1 hour after opening.
3. Place the test card on a clean and level surface.

Materials Provided

1.NEGATIVE RESULT:
If only the C line develops, the test indicates that no detectable Vibrio Cholerae is present in the specimen. The result is negative or non-reactive.
2. POSITIVE RESULT:
In addition to the presence of the C line, if the T1 line develops, the test indicates the presence of Vibrio Cholerae 01 and if the T2 line develops, the test indicates the presence of Vibrio Cholerae 0139. The result is Vibrio Cholerae positive or reactive.
3. INVALID
If the C line does not develop, the assay is invalid regardless of color development of the T1 line and T2 line as indicated below. Repeat the assay with a new device.

 Tuberculosis IgG/IgM Rapid Detection Kit
Tuberculosis IgG/IgM Rapid Detection Kit Tuberculosis IgGIgM Rapid Detection Kit (Colloidal Gold Method)
Tuberculosis IgGIgM Rapid Detection Kit (Colloidal Gold Method) Helicobacter Pylori (H.pylori) IgG/ IgM Test Kit (Colloidal Gold Method)
Helicobacter Pylori (H.pylori) IgG/ IgM Test Kit (Colloidal Gold Method) Typhoid IgG/IgM Test Kit (Colloidal Gold Method)
Typhoid IgG/IgM Test Kit (Colloidal Gold Method) Human Rotavirus Antigen Test Kit (Colloidal Gold)
Human Rotavirus Antigen Test Kit (Colloidal Gold) Enterovirus 71 (EV71)-IgM Detection Kit (Colloidal Gold Method)
Enterovirus 71 (EV71)-IgM Detection Kit (Colloidal Gold Method)