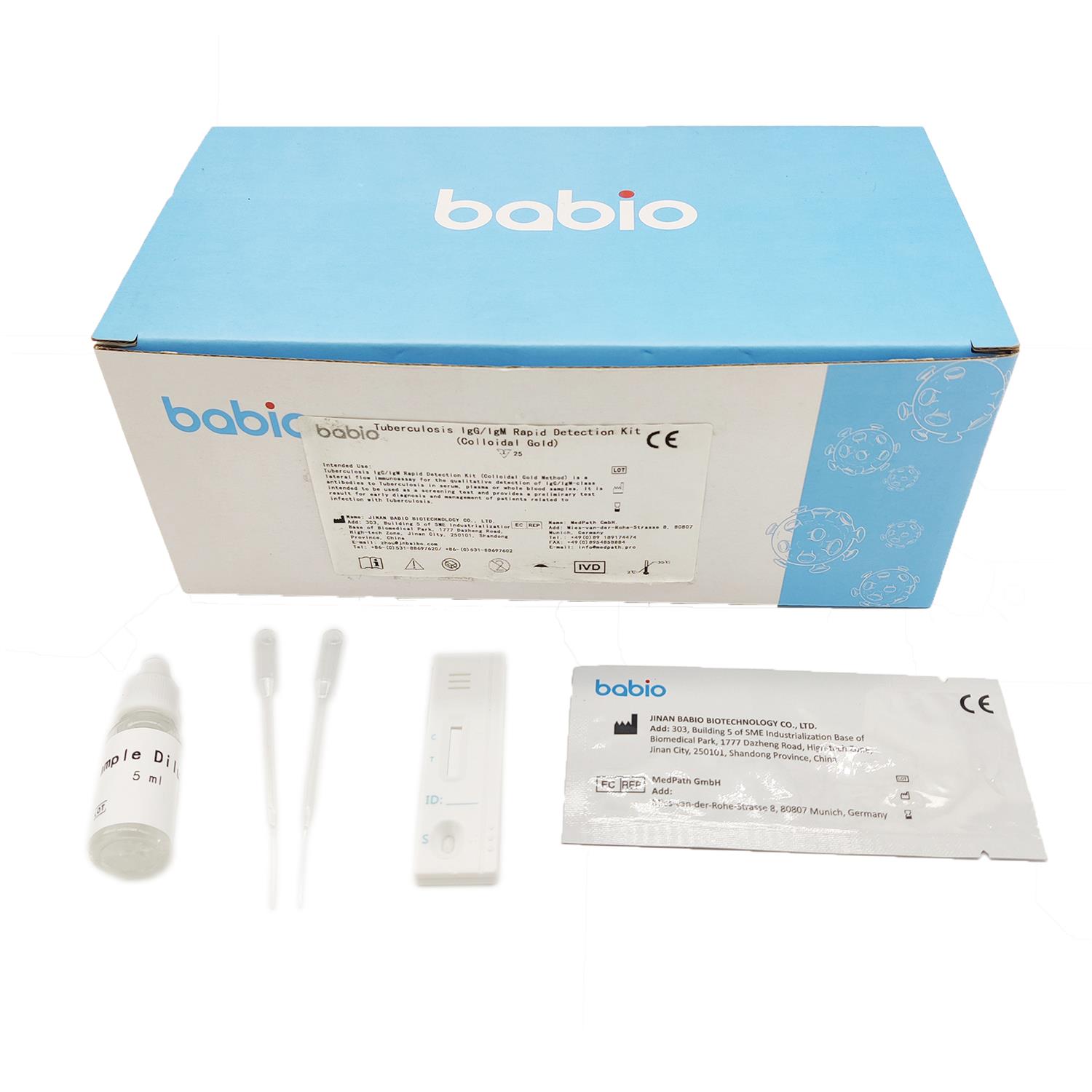
Intended Use
Tuberculosis is a chronic infectious disease caused by Mycobacterium tuberculosis infection with an incubation period of 4 to 8 weeks, 80% of which occur in the lungs. The disease is mainly transmitted through the respiratory tract. When the patient coughs, sneezes, speaks loudly or spit, the droplets with Mycobacterium tuberculosis are excreted from the body, forming microscopic droplets that float in the air and are inhaled by others to cause infection.

If the sample contains an IgG/IgM antibody of Tuberculosis, the antibody will bind to the colloidal gold-labeled Tuberculosis antigen, and the immune complex will be captured by the monoclonal anti-human IgG/IgM antibody immobilized on the nitrocellulose membrane to form a purple/red T line , showing that the sample is positive for IgG/IgM antibody.
Test Procedure
Step1: Allow the test device, buffer, specimen to equilibrate to room temperature (15-30℃) prior to testing.
Step2: Remove the test device from the sealed pouch. Place the test device on a clean, flat surface.
Step3: Label the device with specimen number.
Step4: Using a Disposable Dropper, transfer serum, plasma or whole blood. Hold the dropper vertically and transfer 1 drop of specimen (approximately 10μl) to the specimen well(S) of the test device, and immediately add 2 drops of test buffer (approximately 70-100μl). Make sure there are no air bubbles.
Step5: Set up a timer. Read the results in 15 minutes.
Do not interpret the result after 20 minutes. To avoid confusion, discard the test device after interpreting the result. If you need to store it for a long time, please take a photo of the result.
Results


 Vibrio Cholerae Antigen Detection Kit (Colloidal Gold Method)
Vibrio Cholerae Antigen Detection Kit (Colloidal Gold Method) Tuberculosis IgGIgM Rapid Detection Kit (Colloidal Gold Method)
Tuberculosis IgGIgM Rapid Detection Kit (Colloidal Gold Method) Helicobacter Pylori (H.pylori) IgG/ IgM Test Kit (Colloidal Gold Method)
Helicobacter Pylori (H.pylori) IgG/ IgM Test Kit (Colloidal Gold Method) Typhoid IgG/IgM Test Kit (Colloidal Gold Method)
Typhoid IgG/IgM Test Kit (Colloidal Gold Method) Human Rotavirus Antigen Test Kit (Colloidal Gold)
Human Rotavirus Antigen Test Kit (Colloidal Gold) Enterovirus 71 (EV71)-IgM Detection Kit (Colloidal Gold Method)
Enterovirus 71 (EV71)-IgM Detection Kit (Colloidal Gold Method)