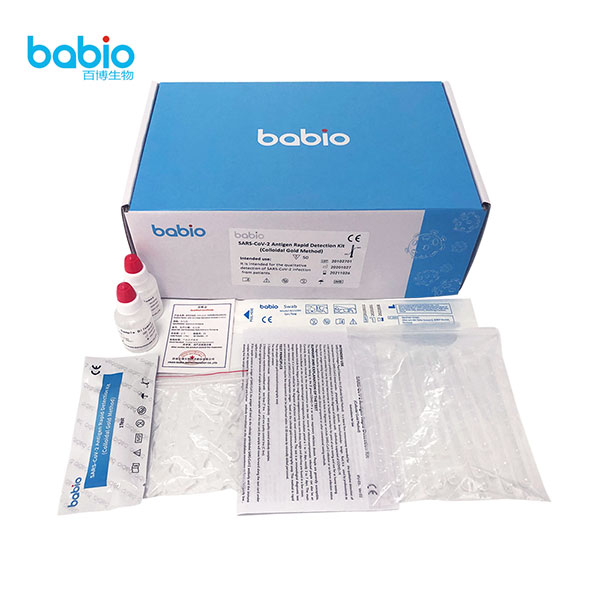
SARS-CoV-2 Antigen Rapid Detection testing kits (Colloidal Gold Method) is a lateral flow immunoassay Medical Diagnostic Reagents. intended for the qualitative detection of nucleoprotein from SARS-CoV-2 innasal swabs,pharyngeal swabs,sputum,bronchoalveolar,lavage fluid.SARS-CoV-2 Antigen Rapid Detection testing kits (Colloidal Gold Method) is a lateral flow immunoassay Medical Diagnostic Reagents. intended for the qualitative detection of nucleoprotein from SARS-CoV-2 innasal swabs, pharyngeal swabs, sputum, bronchoalveolar, lavage fluid. Antigen Test is divided into saliva antigen rapid and Antigen Swab test. Babio antigen kits are of high quality and excellent price. Your inquiry is welcome!






 Combo of SARS-COV-2 / FLU A and B Antigen Rapid Test Kit
Combo of SARS-COV-2 / FLU A and B Antigen Rapid Test Kit Combo of SARS-COV-2 / FLU A and B / RSV Antigen Rapid Test Kit
Combo of SARS-COV-2 / FLU A and B / RSV Antigen Rapid Test Kit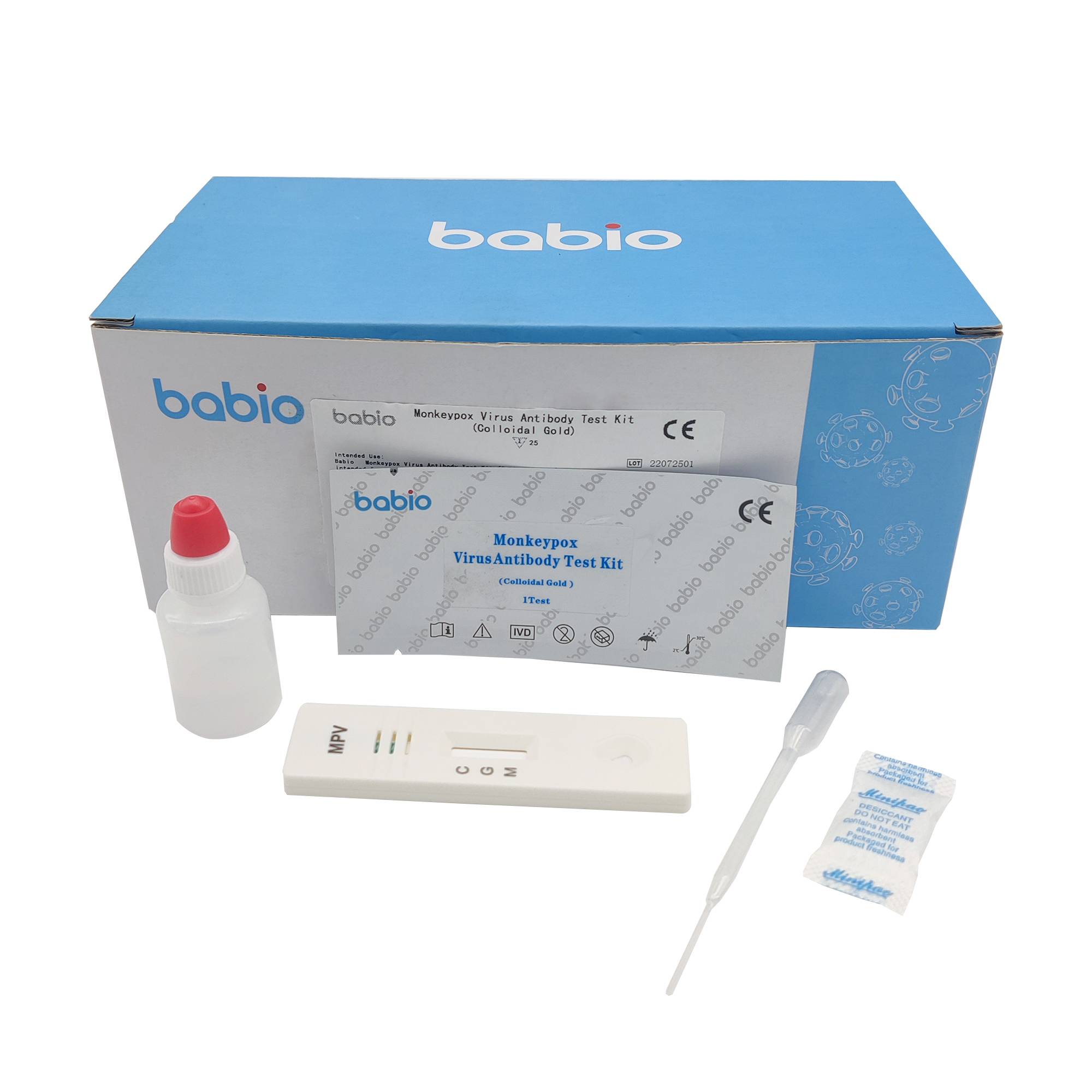 Monkeypox Virus Antibody Test Kit (Colloidal Gold)
Monkeypox Virus Antibody Test Kit (Colloidal Gold)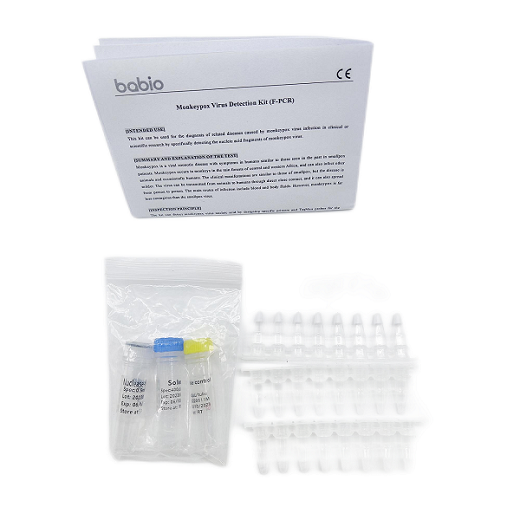 Monkeypox Virus Detection Kit (F-PCR)
Monkeypox Virus Detection Kit (F-PCR)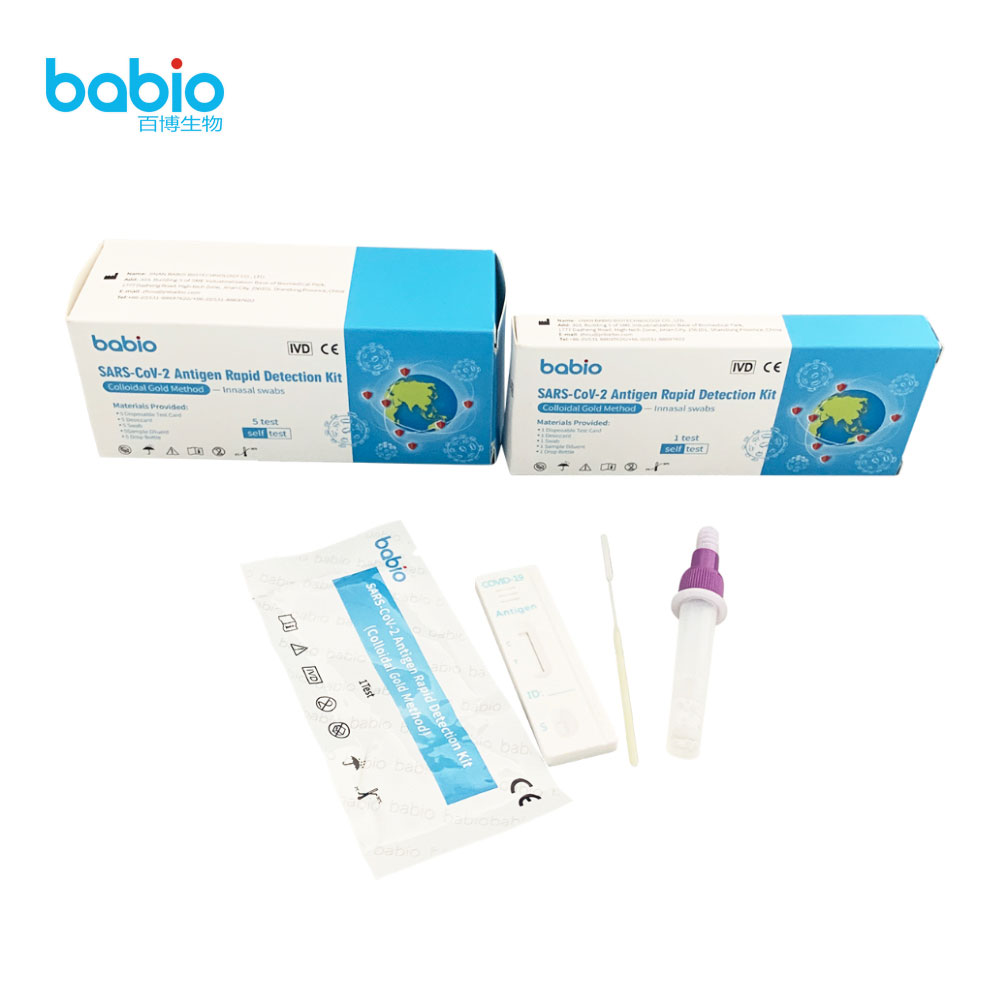 SARS-CoV-2 Antigen Rapid Detection kit with innasal swabs
SARS-CoV-2 Antigen Rapid Detection kit with innasal swabs SARS-CoV-2 Antigen Detection Kit (Latex immunochromatography)
SARS-CoV-2 Antigen Detection Kit (Latex immunochromatography)