
The Combo of SARS-COV-2 / FLU A and B / RSV Antigen Rapid Test Kit is a versatile diagnostic tool designed to detect viral infections quickly. It covers three critical areas: COVID-19, influenza (Flu A and B), and respiratory syncytial virus (RSV). By utilizing specific antigens, this test provides rapid results, allowing healthcare professionals to make timely decisions. Whether in clinics, hospitals, or point-of-care settings, this kit offers a comprehensive solution for identifying these contagious viruses.
INTENDED USE
SARS-CoV-2,FLU A and B,Respiratory syncytial virus are common source of infection that causes respiratory diseases. The symptoms caused by these three viruses are very similar, mainly in headache, fatigue, fever, cough, nasal congestion, and sore throat. It is extremely difficult to judge which virus is caused by the symptoms.
Babio ®Combo of SARS-COV-2 / FLU A and B / RSV Antigen Rapid Test Kit (Colloidal Gold Method)can provide rapid detection of SARS-COV-2 and/or influenza A and/or B and/or RSV viral antigens.It can provides an instant test result in 15 minutes by minimally skilled personnel without the use of laboratory equipment.
TEST PRINCIPLCE
This kit adopts colloidal gold-immunochromatography assay .
SARS-COV-2:
The test card contains:
1. Colloidal gold-labeled anti SARS-CoV-2 monoclonal antibody and quality control antibody complex.
2. Nitrocellulose membranes immobilized with test lines (T line)and one quality control line (C line).
When an appropriate amount of sample is added to the sample well of the test card, the sample will move forward along the test card under capillary action.
If the sample contains an antigen of SARS-CoV-2, the antigen will bind to the colloidal gold-labeled SARS-CoV-2 antibody, and the immune complex will be captured by the monoclonal anti-human antibody immobilized on the nitrocellulose membrane to form a burgundy line , showing that the sample is positive for antigen.
Influenza A/B
The test card contains:
1. Colloidal gold-labeled anti-Influenza A and B monoclonal antibody and quality control antibody complex.
2. Nitrocellulose membranes immobilized with test lines (T1 line and T2 line)and one quality control line (C line).The T1 line is pre-coated with anti-Influenza A antibody, the T2 line is pre-coated with anti-Influenza B antibody, and the C line is pre-coated with a control line antibody.
The Influenza antigen is firstly extracted from the specimen with extraction buffer. The antigen extracts contact the test strip and then migrate by capillary action across the test strip. Influenza A antigen, if presents in the extract, will bind to the antibody conjugates. The immunocomplex is then captured on the membrane by the pre-coated anti-Influenza A antibodies, forming a burgundy colored T1 line, indicating an Influenza A positive test result.
Influenza B antigen, if present in the extract, will bind to the antibody conjugates. The immunocomplex is then captured on the membrane by the pre-coated anti-Influenza B antibodies, forming a burgundy colored T2 line, indicating Influenza B positive test result.
The test contains an internal control (C line) which should exhibit a burgundy colored line of the of the control antibodies regardless of color development on any of the test lines. If the C line does not develop, the test result is invalid and the specimen must be retested with another device.
RSV:
The test card contains:
1. Colloidal gold-labeled anti RSV monoclonal antibody and quality control antibody complex.
2. Nitrocellulose membranes immobilized with test lines (T line)and one quality control line (C line).
When an appropriate amount of sample is added to the sample well of the test card, the sample will move forward along the test card under capillary action.
If the sample contains an antigen of RSV, the antigen will bind to the colloidal gold-labeled RSV antibody, and the immune complex will be captured by the monoclonal anti-human antibody immobilized on the nitrocellulose membrane to form a burgundy line , showing that the sample is positive for antigen.
Materials Provided:
|
component |
1T/box |
2T/box |
5T/box |
20T/box |
25T/box |
50T/box |
|
Test Card |
1 |
2 |
5 |
20 |
25 |
50 |
|
Swab |
1 |
2 |
5 |
20 |
25 |
50 |
|
Sample Diluent |
500ul*1 |
500ul*2 |
500ul*5 |
500ul*20 |
500ul*25 |
500ul*50 |
|
Manual |
1 |
1 |
1 |
1 |
1 |
1 |
SHELF LIFE AND STORAGE
1. The original packaging should be stored in a dry place at 2-30°C and protected from light.
2. The shelf life of the test kit is 2 year from date of manufacture. Refer to the product labels for stated expiration date.
3. The original packaging can be transported at 2-37℃ for 20 days.
4. After opening the inner package, the test card will become invalid due to moisture absorption, please use it within 1 hour.
TEST PROCEDURE
1. Open the packaging box, take out the inner package and let it equilibrate to room temperature.
2. Remove the test card from sealed pouch and use within 1 hour after opening.
3. Place the test card on a clean and level surface.

INTERPRETATION OF ASSAY RESULT
1. NEGATIVE:
SARS-COV-2 / FLU A and B:If only the quality control line C appears, and the test lines T/T1/T2 are not burgundy, it indicates that no antigen is detected, and the result is negative. Due to the limitation of detection sensitivity, negative results may be caused by antigen concentrations lower than the analytical sensitivity of the product.
2. POSITIVE:
SARS-COV-2 and RSV :If both the quality control line C and the test line T appear , it indicates that antigen is detected.Samples with positive results should be confirmed with alternative testing method(s) and clinical findings before a diagnosis is made.
FLU A and B:
In addition to the presence of the C line, if the T1 line develops, the test indicates the presence of Influenza A virus. The result is Influenza A positive or reactive.
In addition to the presence of the C line, if only the T2 line develops, the test indicates the presence of Influenza B virus. The result is Influenza B positive or reactive.
In addition to the presence of the C line, if both the T1 and T2 lines develop, the test indicates the presence of both Influenza A virus and Influenza B virus. The result is Influenza A and B positive or reactive.
3. INVALID:
If the quality control line C is not displayed, the test result is invalid regardless of whether there is a burgundy test line, and it should be tested again.
Repeat the test using remaining sample or new sample, if results are not clear.
If the test repeated fail to produce a result, discontinue using the kit and contact the manufacture.
【Product Advantage】
Comprehensive Coverage: The kit simultaneously detects SARS-CoV-2 (COVID-19), influenza A and B viruses, and RSV.
Rapid Results: Within minutes, the test provides accurate results, aiding in prompt patient management.
Ease of Use: The test is user-friendly, making it suitable for various healthcare settings.
Early Detection: Identifying infections early helps prevent further spread and ensures timely treatment.
Reliable Performance: The test’s sensitivity and specificity meet clinical standards, enhancing diagnostic confidence.
Convenient Packaging: Each kit includes all necessary components for efficient testing.
 Combo of SARS-COV-2 / FLU A and B Antigen Rapid Test Kit
Combo of SARS-COV-2 / FLU A and B Antigen Rapid Test Kit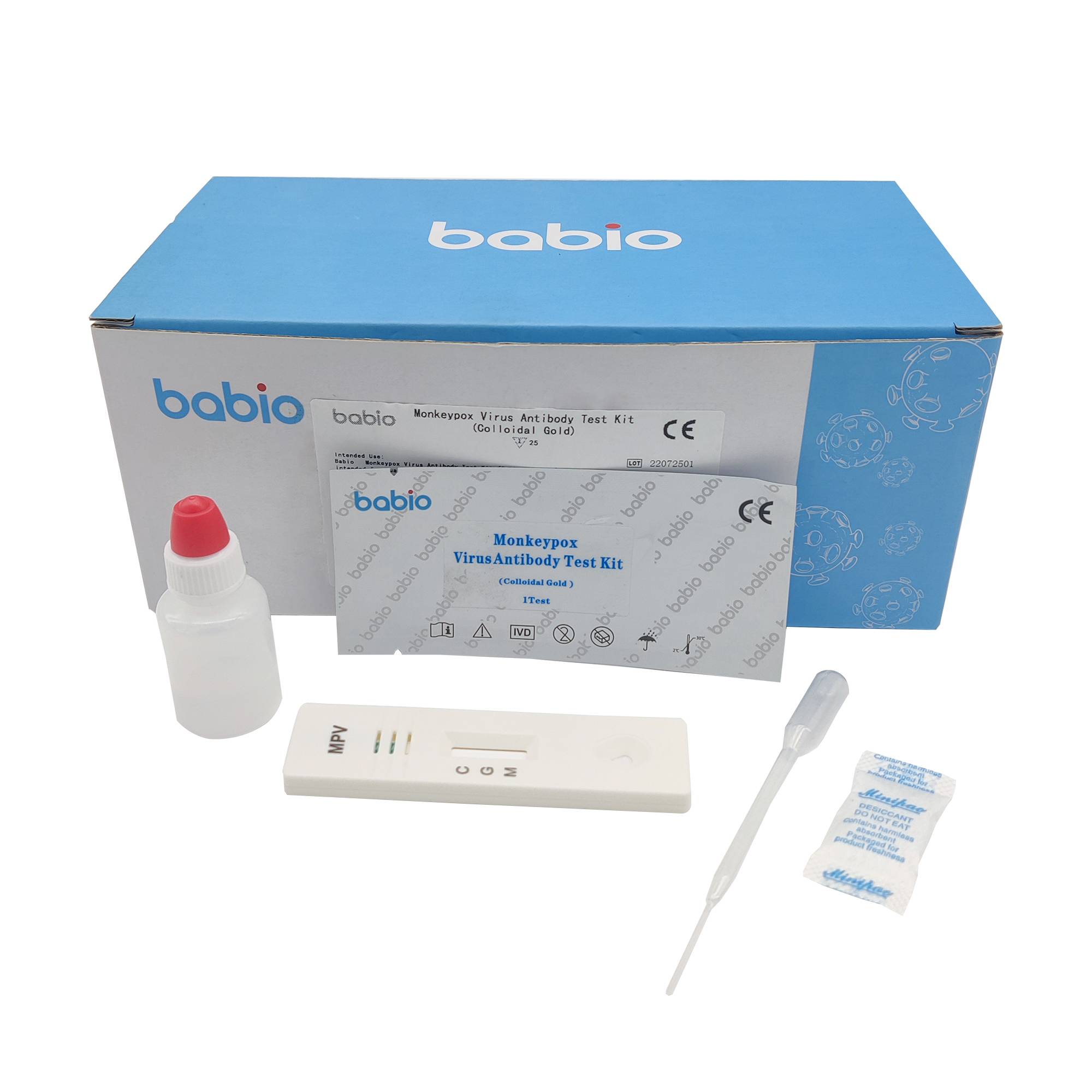 Monkeypox Virus Antibody Test Kit (Colloidal Gold)
Monkeypox Virus Antibody Test Kit (Colloidal Gold) Monkeypox Virus Detection Kit (F-PCR)
Monkeypox Virus Detection Kit (F-PCR)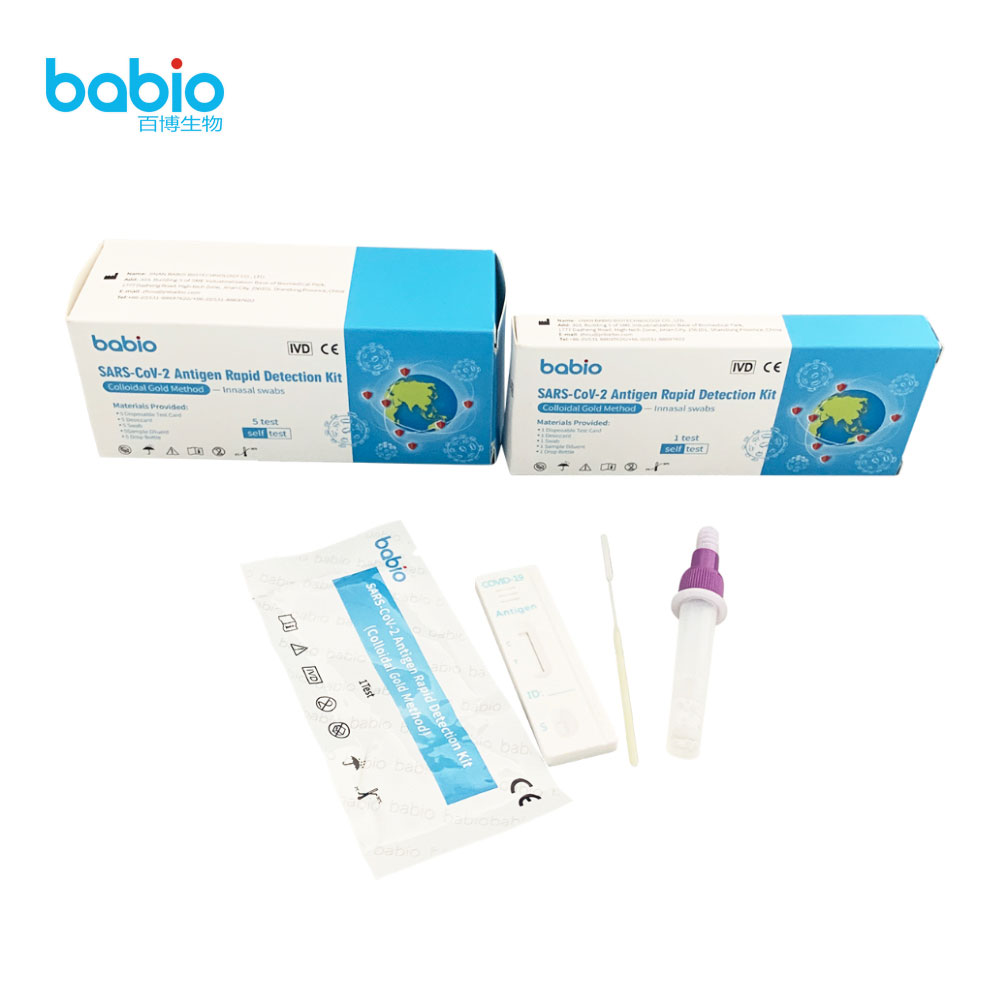 SARS-CoV-2 Antigen Rapid Detection kit with innasal swabs
SARS-CoV-2 Antigen Rapid Detection kit with innasal swabs SARS-CoV-2 Antigen Detection Kit (Latex immunochromatography)
SARS-CoV-2 Antigen Detection Kit (Latex immunochromatography)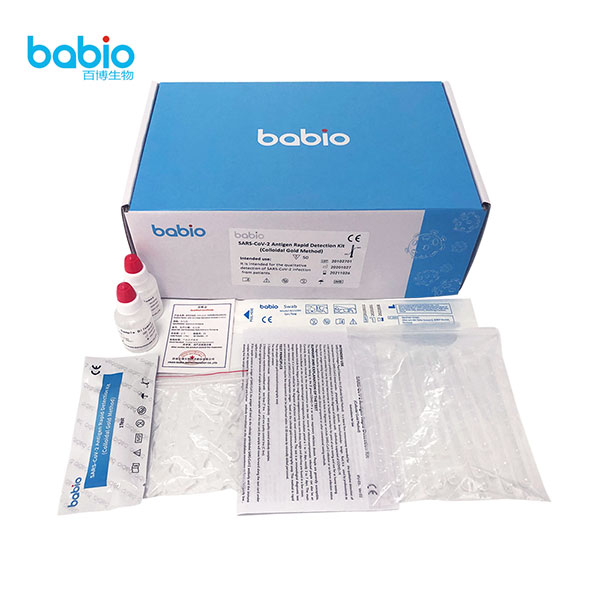 SARS-CoV-2 Antigen Rapid Detection testing kits (Colloidal Gold Method)
SARS-CoV-2 Antigen Rapid Detection testing kits (Colloidal Gold Method)