
SARS-CoV-2 Antigen Detection Kit (Latex immunochromatography)
Instructions for Use
SARS-CoV-2 Antigen Detection Kit(Latex immunochromatography)is a lateral flow immunoassay intended for the qualitative detection of nucleoprotein from SARS-CoV-2 innasal swabs,pharyngeal swabs,sputum,bronchoalveolar,lavage fluid. It is used by professionals as a test and provides a preliminary test result to aid in the diagnosis of infection with individuals suspected of COVID-19.
This test is only provided for use by clinical laboratories or to health care workers for point of care testing, and not for at home testing.
Results from antigen testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status. The diagnosis should be confirmed in combination with clinical symptoms or other conventional testing methods.
Summary And Explanation of The Test
The novel coronaviruses belong to the β genus.COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection;asymptomatic infected people can also be an infectious source.Based on the current epidemiological investigation,the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough.Nasal congestion,runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Antigen detection is a common method for the diagnosis of infection with novel coronavirus. This test is an immunological diagnostic test used for detection of SARS-CoV-2 nucleoprotein antigen based on the Latex-immunochromatography assay. This method is rapid and convenient to use and requires few equipment.It can be performed within 15-20 minutes by minimally skilled personnel.
Test Principlce
This kit adopts Latex immunochromatography assay .
The test card contains:
1. mouse nucleoprotein monoclonal antibody and quality control antibody complex labelled with latex microspheres.
2. Nitrocellulose membranes immobilized with test lines (T line)and one quality control line (C line).
When an appropriate amount of sample is added to the sample well of the test card, the sample will move forward along the test card under capillary action.
If the sample contains an antigen of SARS-CoV-2, the antigen will bind to the latex microspheres labeled SARS-CoV-2 antibody, and the immune complex will be captured by the monoclonal anti-human antibody immobilized on the nitrocellulose membrane to form a red line , showing that the sample is positive for antigen.
Test Reagents
The nominal formula for each medium is as follows:
| Diluent | Test Card |
| Water 90%-99%Sodium chloride 0.1%-1%Sodium citrate 0.1%-0.5%Tween-20 0.1%-1%Sucrose 0.1%-1%Trehalose 0.1%-1%Proclin-300 0.01%-1%PEG20000 0.01%-1%Disodium phosphate 0.0001-1%Sodium dihydrogen phosphate 0.0001-1% | BoraxMESNHSEDCMouse anti |
Reagents And Materials Provided
Materials Provided:
| Component Name | 1T/box | 20T/box | 25T/box | 50T/box | 100T/box |
| Disposable Test Card | 1 | 20 | 25 | 50 | 100 |
| Desiccant | 1 | 20 | 25 | 50 | 100 |
| Swab | 1 | 20 | 25 | 50 | 100 |
| Sample Diluent | 500ul/tube×1 | 12ml/bottle×1 | 15ml/ bottle×1 | 15ml/ bottle×2 | 15ml/ bottle×4 |
| Disposable Plastic Dropper | 1 | 20 | 25 | 50 | 100 |
| tube | 1 | 20 | 25 | 50 | 100 |
Or
| Component Name | 1T/box | 20T/box | 25T/box | 50T/box | 100T/box |
| Disposable Test Card | 1 | 20 | 25 | 50 | 100 |
| Desiccant | 1 | 20 | 25 | 50 | 100 |
| Swab | 1 | 20 | 25 | 50 | 100 |
| Sample Diluent | 500ul/tube×1 | 12ml/bottle×1 | 15ml/ bottle×1 | 15ml/ bottle×2 | 15ml/ bottle×4 |
| Drop Bottle | 1 | 20 | 25 | 50 | 100 |
Or
| Component Name | 1T/box | 20T/box | 25T/box | 50T/box | 100T/box |
| Disposable Test Card | 1 | 20 | 25 | 50 | 100 |
| Desiccant | 1 | 20 | 25 | 50 | 100 |
| Disposable Device | 1 | 20 | 25 | 50 | 100 |
| Biosafety bag | 1 | 20 | 25 | 50 | 100 |
Note:One disposable device contains one swab and 0.5ml sample diluent.
Specification:1T/box,20T/box,25T/box,50T/box,100T/box
Materials Required But Not Provided
1.PPE such as gloves, masks, lab coats, and eye protection
2.Biohazard Waste Container
3.Tube holder
Warnings And Precautions
1. For emergency and use by medical or health professionals only at designated point of care facilities.
2. Read the package insert in its entirety prior to performing the test. Failure to follow the package insert instructions may result in an invalid test result.
3. Wear appropriate protective clothing when handling and processing specimens. Wash hands thoroughly after handling specimen.
4. Handle specimens as if they contain infectious agents in accordance to standardized procedures,and the US CDC Universal Precautions.
5. Do not use it if the tube/pouch is damaged or broken.
6. Test is for single use only. Do not re-use under any circumstances.
7. Humidity and temperature can adversely affect results.
8. Follow storage recommendations listed on the product labels. Storage and handling outside of these conditions may adversely affect product.
9. Do not use product after indicated expiration date.
10. Dispose of all samples and used test components in appropriately approved and labeled biohazard waste containers.
Shelf Life And Storage
1. The original packaging should be stored in a dry place at 2-30°C and protected from light.
2. The shelf life of the test kit is 1 year from date of manufacture. Refer to the product labels for stated expiration date.
3. After opening the inner package, the test card will become invalid due to moisture absorption, please use it within 1 hour.
4. The original packaging should be transported at 2-37℃ for 20 days.
Specimen Collection And Handling
This test can be performed using either humaninnasal swabs,pharyngeal swabs,sputum,bronchoalveolar,lavage,fluid etc. samples can be collected using components provided with the test and should be tested immediately. Please see diagram in Test Procedure section.
Test Procedure
1. Open the packaging box, take out the inner package and let it equilibrate to room temperature.
2. Remove the test card from sealed pouch and use within 1 hour after opening.
3. Place the test card on a clean and level surface.

|

|

|
| ①Specimen from SARS-CoV-2 innasal swabs,pharyngeal swabs,sputum,bronchoalveolar,lavage fluid. | ②drop in 500ul (about 9-10 drops ) Sample Diluent from the drop bottle to the tube.Place the patient swab sample into the Tube. Roll the swab at least 3 times while pressing the head against the bottom and side of the Tube. | ③Roll the Swab head against the inside of the Tube as you remove it. Dispose of the used Swab in your biohazard waste. |

|

|

|
| ④Fill the provided Small, Clear Disposable Plastic Dropper with patient sample from the tube or Put the lid on the dropper bottle. | ⑤Drop 60-100ul sample (2-3 drops) into the test card.NOTE: Do not pour sample from the Tube. | ⑥Read the result at 15 minutes.The result is valid within 15-20 minutes. It must be repeated |
Or

|

|

|
| ①specimen from SARS-CoV-2 innasal swabs,pharyngeal swabs,sputum,bronchoalveolar,lavage,fluid. | ②Break the inner safety core and squeeze the liquid into the bottom of the tube. | ③Squeeze the tip of a swab to dilute the specimen |

|

|

|
| ④Twisting the tail cap cover dripper | ⑤Squeeze about 60-100ul(2-3drops) specimen diluent onto the reagent card | Read the result at 15 minutes.The result is valid within 15-20 minutes. It must be repeated |
Quality Control
1. The test card includes an internal procedural control. This control confirms that sufficient specimen volume and technique have been applied.
2. Control standards are not provided with this kit.
3. It is recommended to follow good laboratory practice including adding positive and negative controls in order to verify proper test performance.
INTERPRETATION OF ASSAY RESULT
1. Negative:
If only the quality control line C appears, and the test lines T are not red, it indicates that no antigen is detected, and the result is negative. Due to the limitation of detection sensitivity, negative results may be caused by antigen concentrations lower than the analytical sensitivity of the product.
2. Positive:
If both the quality control line C and the test line T appear , it indicates that antigen is detected.Samples with positive results should be confirmed with alternative testing method(s) and clinical findings before a diagnosis is made.
3. Invalid:
If the quality control line C is not displayed, the test result is invalid regardless of whether there is a red test line, and it should be tested again.
Repeat the test using remaining sample or new sample, if results are not clear.
If the test repeated fail to produce a result, discontinue using the kit and contact the manufacture.
PERFORMANCE CHARACTERISTICS
Cross Reactivity
SARS-CoV-2 Antigen Detection Kit(Latex immunochromatography) has been tested for Influenza A H1N1 antigen, Influenza A H3N2 antigen, Influenza B antigen, Adenovirus antigen, Mycoplasma antigen , Respiratory Syncytial antigen, Staphylococcus aureus antigen,Streptococcus pneumonia antigen positive specimens. The results showed no cross-reactivity.
Interference
Add a certain concentration of pathogens to the clinically negative samples, and the test results should have no interference reaction. The added pathogens are shown in the following table:
| pathogens | concentration | pathogens | concentration |
| Human coronavirus 229E | 1,0×10 6 pfu / ml | Respiratory syncytial virus | 1,0×10 6 pfu / ml |
| Human coronavirus OC43 | 1,0×10 6 pfu / ml | Adenovirus | 1,0×10 6 pfu / ml |
| Human coronavirus NL63 | 1,0×10 6 pfu / ml | Influenza A H1N1 | 1,0×10 6 pfu / ml |
| coronavirus MERS | 1,0×10 6 pfu / ml | Influenza B | 1,0×10 6 pfu / ml |
Limitations of Test
1. This product is for qualitative assessment of SARS-CoV-2 antigen only.
2. This test is only provided for use by clinical laboratories or to health care workers for point of care testing, and not for at home testing.
3. Results from antigens testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status. The diagnosis should be confirmed in combination with clinical symptoms or other conventional testing methods.
4. Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
5. A negative or non-reactive result can occur if the quantity of antigen for the SARS-CoV-2 virus present in the specimen is below the detection limit of the assay.
6.This test can detects SARS-CoV and SARS-CoV-2 no matter the virus is viable (live) or non-viable. Test performance depends on the amount of virus (antigen) in the sample ,but it does not necessarily correlate to SARS-CoV-2 antigen titer in the specimen.
7.A negative test result may occur if the level of antigen is below the detection limitation or if the sample was collected or transported improperly.
8.Failure to follow the Test Procedure may adversely affect test performance and/or invalidate the test result.
References
1. Chaolin Huang, Yeming Wang, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet.2020;VOL395:497-506.
2. Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. 24 January 2020. New England Journal of Medicine.
3. Lamarre A, Talbot PJ. Effect of pH and temperature on the infectivity of human coronavirus 229E. Canadian Journal of Microbiology. 1989;35(10):972-4.
4. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. The Lancet. 24 January 2020.
 Combo of SARS-COV-2 / FLU A and B Antigen Rapid Test Kit
Combo of SARS-COV-2 / FLU A and B Antigen Rapid Test Kit Combo of SARS-COV-2 / FLU A and B / RSV Antigen Rapid Test Kit
Combo of SARS-COV-2 / FLU A and B / RSV Antigen Rapid Test Kit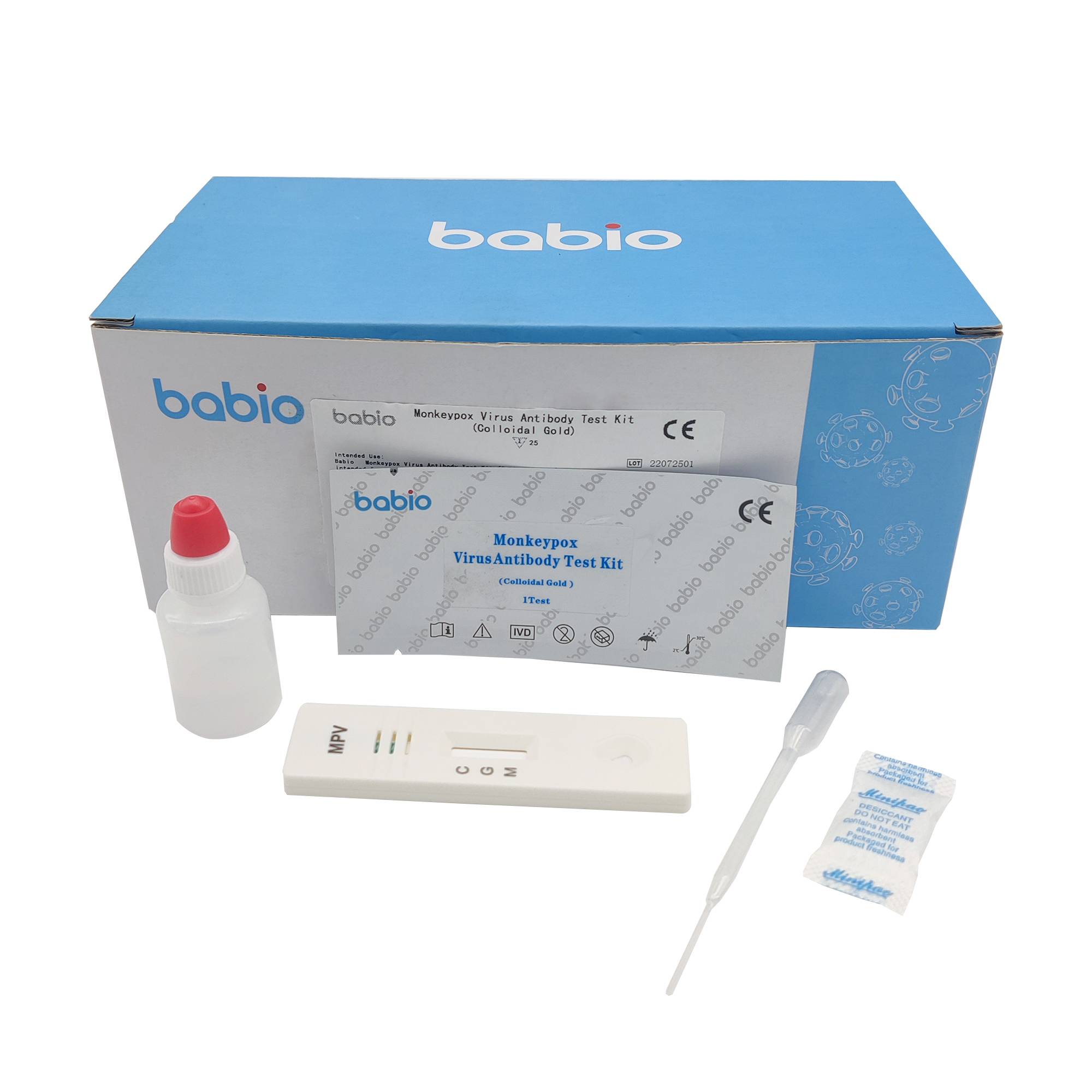 Monkeypox Virus Antibody Test Kit (Colloidal Gold)
Monkeypox Virus Antibody Test Kit (Colloidal Gold) Monkeypox Virus Detection Kit (F-PCR)
Monkeypox Virus Detection Kit (F-PCR)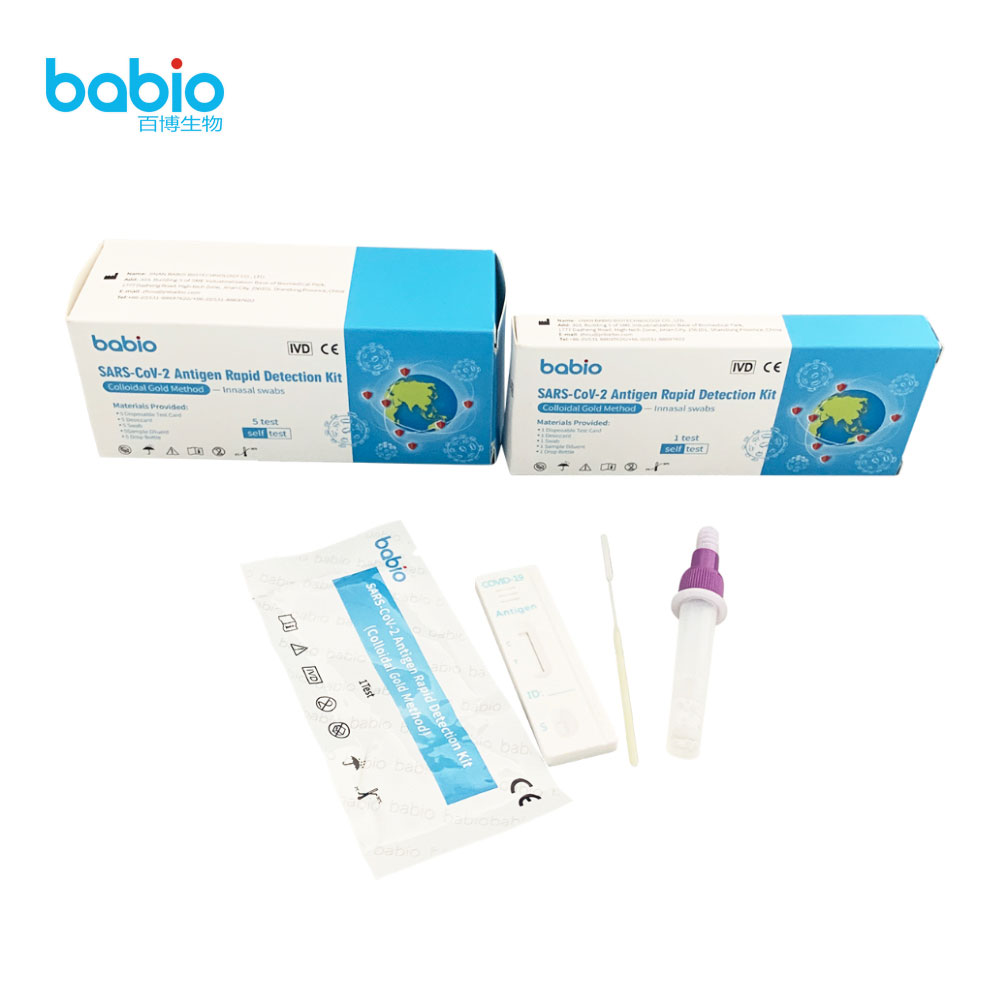 SARS-CoV-2 Antigen Rapid Detection kit with innasal swabs
SARS-CoV-2 Antigen Rapid Detection kit with innasal swabs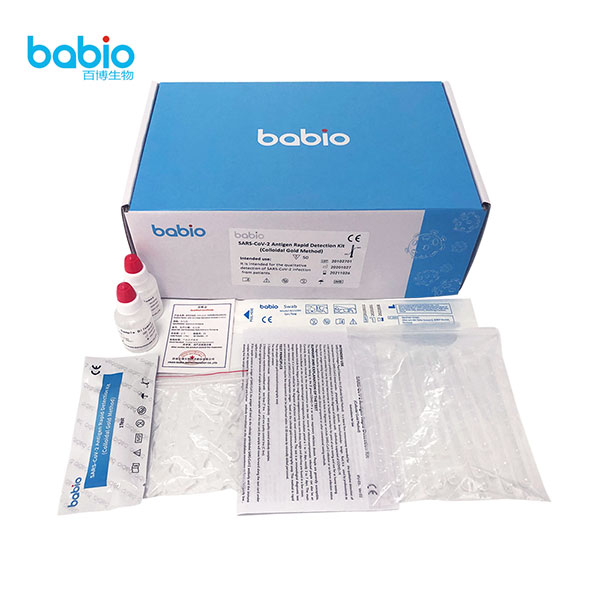 SARS-CoV-2 Antigen Rapid Detection testing kits (Colloidal Gold Method)
SARS-CoV-2 Antigen Rapid Detection testing kits (Colloidal Gold Method)