Global Surge in Foodborne Illness Cases Highlights the Importance of Reliable Transport Media - Babio’s Modified Cary-Blair Transport Media Gains Global Attention
In the wake of rising global outbreaks of foodborne illnesses caused by Salmonella, Shigella, Campylobacter, and Vibrio species, the demand for reliable and standardized stool specimen transport solutions has surged dramatically. Recent data from international health agencies, including the CDC, WHO, and EFSA, highlights the increasing incidence of bacterial gastroenteritis across Southeast Asia, the Middle East, Africa, and parts of Europe. These alarming trends have pushed healthcare systems, diagnostic laboratories, and food safety authorities to strengthen their testing and surveillance infrastructure.
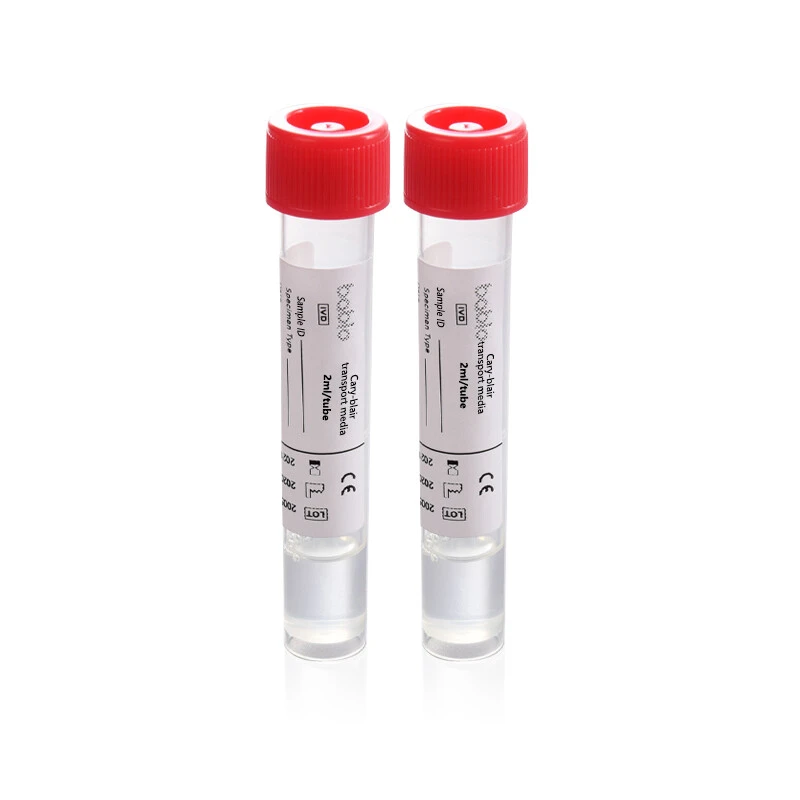
At the heart of effective microbial testing lies the critical step of sample collection and transport. This is where Babio Biotech, a leading Chinese manufacturer specializing in microbiological and diagnostic solutions, steps in with its Modified Cary-Blair Transport Media. Designed for safe, stable, and reliable transport of enteric pathogens, Babio’s product has been recognized as an essential tool in ensuring accurate diagnosis and timely outbreak response.
Why Modified Cary-Blair Transport Media Matters Now More Than Ever
- Global Increase in Diarrheal Diseases: searches for “Salmonella symptoms,” “Shigella infection treatment,” and “Campylobacter in food” have spiked significantly over the past 12 months.
- International Travel and Food Trade: The rise in cross-border food trade and post-pandemic travel has increased the risk of bacterial contamination and the spread of enteric infections globally.
- Compliance with International Standards: Laboratories in Europe, Southeast Asia, and the Middle East are aligning their testing protocols with FDA, WHO, and ISO standards, all of which recommend validated transport media like Modified Cary-Blair.
- Demand for Reliable Chinese Suppliers: With global supply chains under strain, hospitals and laboratories are actively searching for certified Chinese manufacturers that offer cost-effective, high-quality products with global compliance. This is where Babio Biotech (https://www.babiocorp.com) excels, offering both affordability and performance assurance.
Features That Make Babio’s Modified Cary-Blair Transport Media Stand Out
✅ Maintains viability of enteric bacteria like Salmonella, Shigella, and Campylobacter for up to 48 hours at ambient temperatures.
✅ Conforms to international quality standards, tested for pH stability and bacterial preservation.
✅ Suitable for rectal swab and stool specimens, supporting routine and outbreak investigations.
✅ Available in 2 mL and 3 mL tubes, ideal for clinical, food safety, and environmental health testing.
Current Market Needs Align with Babio’s Innovation
Babio Biotech ensures that diagnostic laboratories have access to proven transport media solutions that support fast, accurate diagnosis. This not only helps contain outbreaks but also supports public health surveillance programs globally.
With certified manufacturing facilities and a proven export record across Europe, Africa, Southeast Asia, and the Middle East, Babio Biotech is committed to supporting the global healthcare community in the fight against foodborne diseases.
Explore More:
Learn more about Babio Biotech and its full range of diagnostic and microbiology products at https://www.babiocorp.com.

