INTENDED USE
Cary-Blair Transport Media is recommended for collection and shipment of clinical specimens.
SUMMARY AND EXPLANATION
Enteric infections can be caused by different types of bacteria. With such a wide array of pathogens and the need for cost containment, physician input and practice guidelines can help the laboratory determine which tests are appropriate for detecting the etiological agent of diarrhea. Microbiology laboratories should review the local epidemiology of bacterial enterocolitis and implement routine stool culture methods that will allow recovery and detection of all the major pathogens causing most of the cases in their geographic area. All microbiology laboratories should routinely test for the presence of Salmonella spp, Shigella spp, and Campylobacter spp. on all stool cultures.1 One of the routine procedures in the diagnosis of enteric infections involves the collection and safe transportation of rectal swab samples or stool samples. This can be accomplished using the Modified Cary-Blair Transport Media. The medium is designed to maintain the viability of enteric pathogenic bacteria during transit to the testing laboratory.
PRINCIPLES OF THE PROCEDURE
This medium does not contain any nutritional components, allowing for the preservation of specimens in a non-nutritive state for an extended period. The presence of sodium thiogycollate in the medium creates a low oxidation-reduction potential environment,
disodium hydrogen phosphate acts as a buffer, and sodium chloride maintains the osmotic pressure balance of the system and also regulates the permeability of the bacterial cell membrane.
STORAGE
This product is ready for use and no further preparation is necessary. The product can be sealed and stored at 2-25℃ for 18 months until used. Do not overheat. Do not incubate or freeze prior to use.Improper storage will result in a loss of efficacy. Do not use after expiration date.
PRODUCT DETERIORATION
Modified Cary-Blair Transport Media should not be used if (1) there is evidence of damage or contamination to the product, (2)
there is evidence of leakage, (3) the expiration date has passed, (4) the package is open, or (5) there are other signs of deterioration.
EXPIRATION DATE
18 months from the date of manufacture.
SPECIMEN COLLECTION
Rectal swab specimens and stool specimens collected for microbiological investigations which comprise the isolation of enteric
pathogenic bacteria should be collected and handled following published manuals and guidelines.1,7-10 To maintain optimum
organism viability, transport specimens collected using Modified Cary-Blair Transport Media directly to the laboratory, preferably within 2 hours of collection.1,7-12 If immediate delivery or processing is delayed, then specimens should be stored at 2-8 °C and processed within 24 hours.
Specific requirements for the shipment and handling of specimens should be in full compliance with local regulations.3-6Shipping of specimens within medical institutions should comply with internal guidelines of the institution. All specimens should be processed as soon as they are received in the laboratory.
PROCEDURES
Materials Provided: Polypropylene screw-cap tube filled with 2 mL or 3 mLof Modified Cary-Blair Transport Media Specification: 2 ml/tube ,3 ml/tube; 20 pieces/pack, 50 pieces/pack, 100 pieces/pack.
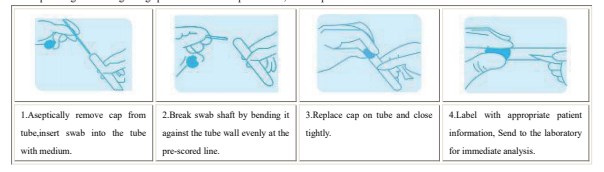
Test Procedurea
Proper specimen collection from the patient is extremely critical for successful isolation and identification of infectious organisms.
For specific guidance regarding specimen collection procedures, consult published reference manuals.
QUALITY CONTROL
Modified Cary-Blair Transport Media applicators are tested to ensure they are non-toxic to enteric pathogenic bacteria.BPX® Modified Cary-Blair Transport Media is tested for pH stability. BPX®Modified Cary-Blair Transport Media is quality control
tested before release for ability to maintain viable enteric pathogenic bacteria at room temperature for specified time points. If aberrant quality control results are noted, patient results should not be reported.
LIMITATIONS OF THE PROCEDURE
1. Condition, timing and volume of specimen collected for culture are significant variables in obtaining reliable culture results. Follow recommended guidelines for specimen collection.
2. This product is intended to be used with the swabs,The use of tubes of medium or swabs from any other source has not been validated and could affect the performance of the product.
3. In the laboratory, wear latex gloves and other protection commensurate with universal precautions when handling clinical specimens.
4. Modified Cary-Blair Transport Media is intended for use as a collection and transport medium for enteric pathogenic bacteria. BPX®Modified Cary-Blair Transport Media cannot be used as enrichment, selective or differential medium.
PERFORMANCE
In an aseptic environment, using a swab to collect samples of Vibrio parahaemolyticus, Salmonella enterica, and Shigella flexneri, store them at 20-25°C for 48 hours. Then transfer the samples to blood agar medium and incubate at 36±1°C for 18-24 hours to observe bacterial viability. The bacteria should grow well.