INTENDED USE

Specification: 1T/box,20T/box,25T/box,50T/box
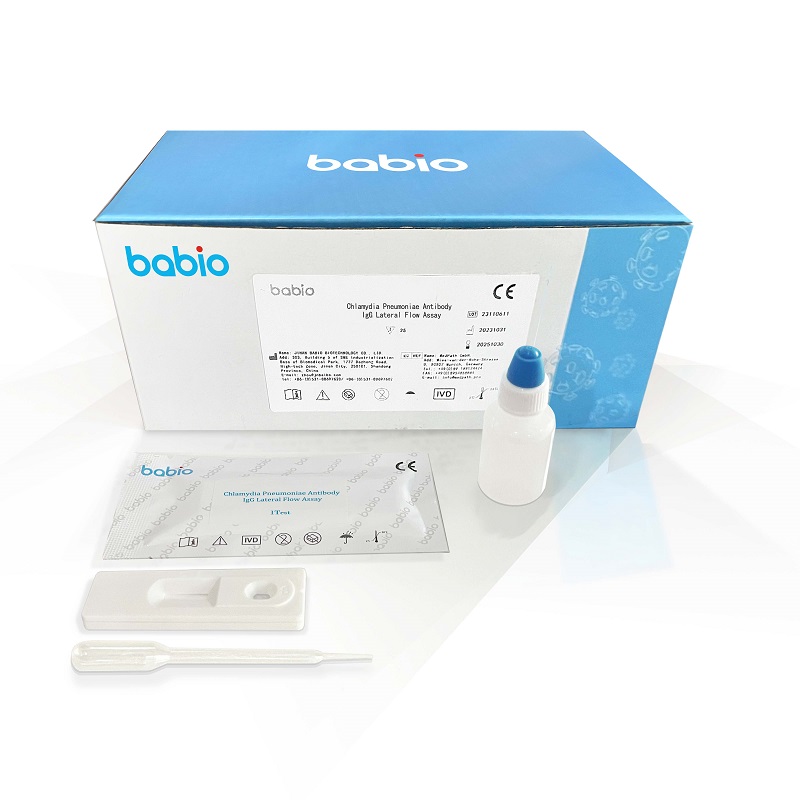
【Specifications and components】
Each box contains 25 test cards, and each test card is separately sealed and packaged with desiccant. Sample diluent 1 bottle, 7ml/ bottle. 1 copy of instruction manual.
【Storage and expiration date】
1. The packaged kit should be stored in a dry place with ventilation from 4 ℃ to 30 ℃, away from light, and prohibited from freezing.
2. Validity: 18 months
【Sample requirement】
This test card is suitable for fresh blood and serum samples. For specimens taken from other parts of the body, the effect is not clear.
1. Specimen collection of serum for testing specimens can be used directly. If clinical isolation of fresh serum is collected for testing samples, the separation of fresh serum samples should be completed within 1 hour, and the storage time should not exceed 48 hours at 4 ℃ for more than 1 hour.
2. Detection methods Tear the aluminum film bag and take out the test plate, place it flat, add 10 μl serum into the sampling hole at the right end of the test plate, and add 100 μl sample diluent. Observe the results of the detection window in the middle of the test card after 3 to 5 minutes, and the observation results are valid within 20 minutes.
【Inspection method】
1. Specimen collection of serum for testing specimens can be used directly. If clinical isolation of fresh serum is collected for testing samples, the separation of fresh serum samples should be completed within 1 hour, and the storage time should not exceed 48 hours at 4 ℃ for more than 1 hour.
2. Detection methods Tear the aluminum film bag and take out the test plate, place it flat, add 10 μl serum into the sampling hole at the right end of the test plate, and add 100 μl sample diluent. Observe the results of the detection window in the middle of the test card after 3 to 5 minutes, and the observation results are valid within 20 minutes.
【Results】
During the effective reaction time of the test sample, if there is Chlamydia pneumoniae IgG antibody in the sample, a red detection line and a red quality control line appear on the reaction membrane; if there is no Chlamydia pneumonia IgG antibody in the sample, only a red quality control line appears on the reaction membrane; if neither accusation line nor detection line appear on the reaction membrane, the test result is invalid. The reaction diagram of test results is shown in the following figure.
positive: there is a red line at T and C of the observation window.
Negative: Only a red line appears in the viewing window C, and no color line appears in the T zone.
Invalid: No color line appears in the observation window T and C, indicating that the test has failed or failed.
